Abstract
Introduction: Despite calls for greater transparency in reporting harms in cancer clinical trials, data on drug toxicity continues to be obscured by incomplete reporting and use of subjective, minimizing language. Subjective terms such as "acceptable" or "tolerable" toxicity are commonly employed in peer-reviewed publications in oncology (Gyawali et al. BMJ 2018). The use of subjective language that minimizes harms in clinical trials can adversely impact informed consent and shared decision-making by overlooking the fact that only individual patients can determine if a therapy is tolerable. The scope of this problem in clinical trials in hematologic malignancies and at medical conferences, however, remains unknown. To further assess this issue, we conducted a systematic review of abstracts presented at ASH annual meetings from 2017-2021 to characterize toxicity reporting and use of subjective, minimizing language in phase 3 clinical trials in acute leukemia.
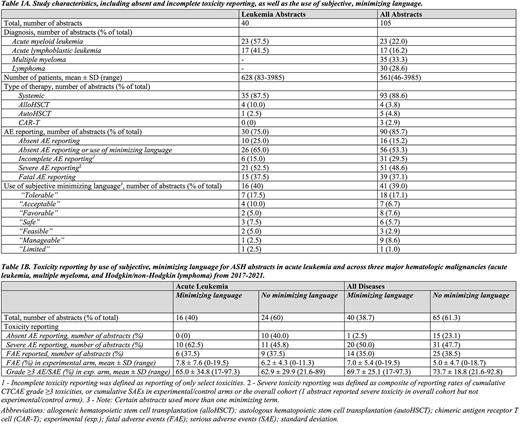
Methods: Following a pilot phase to identify and define minimization terms, we conducted a systematic search of all ASH abstracts from 2017-2021. Phase 3 randomized controlled trials in acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) were included; subset analyses, long-term follow up studies and supportive care trials were excluded. Subjective, minimizing language was defined a priori as use of "acceptable", "tolerable", "manageable", "favorable", "feasible" "safe", and/or "limited" in reference to treatment-related adverse events (AE), which falsely suggested that patients deemed the therapy as such without evaluating patient experiences. Primary toxicity outcome assessment included fatal AE (FAE), serious AE (SAE), and use of common terminology criteria for AE (CTCAE), as well as absent and incomplete AE reporting, defined in Table 1A. Complete severe AE reporting was defined as reporting of cumulative rates (per 100 patients; %) of CTCAE grade ≥3 toxicities or cumulative SAE. An across-disease assessment was performed including phase 3 clinical trials in multiple myeloma and lymphoma from the same time period.
Results: 341 studies in acute leukemia underwent abstract screening, of which 40 met inclusion criteria. Study characteristics are reported in Table 1A. AE reporting was variable across trials. Ten trials (25%) provided no information on AE in their abstract, and 6 (15%) had incomplete AE reporting. Subjective, minimizing language was used to describe AE data in 16 trials (40%), with "tolerable" and "acceptable" being the most frequently used terms, and a total of 26 (65%) studies had either absent AE reporting or used minimizing terms (Table 1A). Minimizing language was seen across a range of different study types, including trials in patients with adult and pediatric leukemia, trials of systemic therapies and stem cell transplantation, and trials in frontline and relapsed/refractory settings. Severe toxicities were reported in 10 of the 16 trials that used minimizing language (62.5%) and FAE in 6 of 16 (37.5%). In experimental arms of trials using minimizing terms, rates of CTCAE grade ≥3 AE or SAE ranged from 17-97.3%, and rates of fatal AE from 0-19.5% (Table 1B). FAE and severe AE rates were similar or numerically higher in abstracts that used minimizing terms compared to those abstracts that did not. A total of 65 abstracts reporting results of randomized clinical trials in multiple myeloma and lymphoma (including Hodgkin and non-Hodgkin lymphoma) were available for across-disease assessment and demonstrated similar high rates of absent and incomplete AE reporting, as well as subjective, minimizing terms, used to describe a wide range of severe AE (Table 1B).
Discussion: This systematic review of ASH abstracts highlights inconsistent, incomplete, and often absent toxicity reporting, as well as frequent use of subjective, minimizing language. The findings suggest that formal toxicity minimization and subjective minimizing terms are common. Conference abstracts often form the first impression of a clinical trial and can have a disproportionate influence on physician perspectives, thus impacting clinical practice. These findings will inform a larger systematic review to inform policy to improve reporting transparency at hematology and oncology conferences.
#co-senior authors
Disclosures
Lyman:Fresenius Kabi: Consultancy; Seattle Genetics: Consultancy; Sandoz: Consultancy; Squibb: Consultancy; Merck: Consultancy; Samsung Bioepis: Consultancy; G1 Therapeutics: Consultancy; Beyond Spring: Consultancy; Amgen: Research Funding. Kuderer:Spectrum: Consultancy; Pfizer: Consultancy; Janssen: Consultancy; Seattle Genetics: Consultancy; Sandoz: Consultancy; G1 Therapeutics: Consultancy; Bristol Myers Squibb: Consultancy; Beyond Spring: Consultancy; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal